Compliance with clinical trial study medication is a common concern. Here’s how sponsors and CROs can use digital technology to help patients stay on track.
Medication compliance, or, more specifically, lack thereof, can affect nearly every aspect of a clinical trial. Patients who fail to follow treatments as instructed can impact studies financially, reduce the reliability of data, and even put their own health at risk.
With 40 percent of patients becoming non-compliant to investigational medical product (IMP) after 150 days, it’s clear that compliance is a widespread problem. To address this challenge, leaders in digital healthcare technology have developed innovative solutions to improve patient compliance. These tools are designed to prompt patients to take their medications as prescribed, and then monitor the results.
As medication adherence rises, so can the efficacy of treatment. Further, when more patients are compliant, there is less pressure on sponsors and CROs to make up for data variability with heightened recruitment efforts. Here are some of the cutting-edge medication adherence tools making their way into healthcare, and how they could impact clinical trials.
Digital Technologies Making an Impact
Healthcare startups and other leaders in digital technology are making important strides in improving IMP adherence. Nomi, for example, is a medication adherence system that collects patient data and identifies patterns to boost adherence. The system sends text messages directly to patients that prompt them to take their medication. Nomi also communicates with patients’ family members, caregivers, and clinical teams to maximize accountability. The system can be used with ingestible capsules, nebulizers, inhalers, injectables, and bottle and cap devices. Nomi has been proven to change patients’ behavior in at least 40 percent of cases.
Another up-and-coming digital tool, the Medocity Enterprise Platform allows healthcare providers to monitor patients between visits. It measures patient-reported data, identifies escalating symptoms, and sends relevant alerts to clinicians. This shared visibility allows different providers to monitor IMP adherence and coordinate care. The platform also offers a telehealth suite that includes digital assistants and video calls and is accessible on hundreds of different devices. The technology has proven to be effective in recent studies, including a trial with Carter Health Psychiatry & Wellness in which it raised patient compliance, satisfaction, and engagement scores to over 90 percent.
Tying these ideas together is Drive, a product developed by HealthPrize. This digital tool is designed to streamline the development of branded patient education and adherence programs. It uses games, behavioral economics, and loyalty marketing to boost patient engagement and improve medication adherence. Programs using this technology have seen a 52 percent improvement in adherence across a range of treatments, conditions, demographics, and delivery methods.
The Impact of Adherence on Clinical Trial Results
While still in its early stages, adherence and compliance technology promises to benefit patients by boosting the efficacy of their treatments and helping them avoid the danger of skipping or doubling doses. What’s more, as studies start to leverage tools like those outlined above to become more streamlined and patient-centric, they are likely to see increased enrollment and engagement, as well as better data and results.
For sponsors and CROs, these tools have the potential to lower overhead costs and shorten recruitment timelines. They can also satisfy a trial’s target statistical outcomes and improve the accuracy of the data it captures. By incorporating innovative technologies like those mentioned above, sponsors and CROs can proactively address common challenges related to compliance, adherence, engagement, and data collection, ensuring patients receive the highest-quality care and potential new treatments are brought to market as quickly and efficiently as possible.





























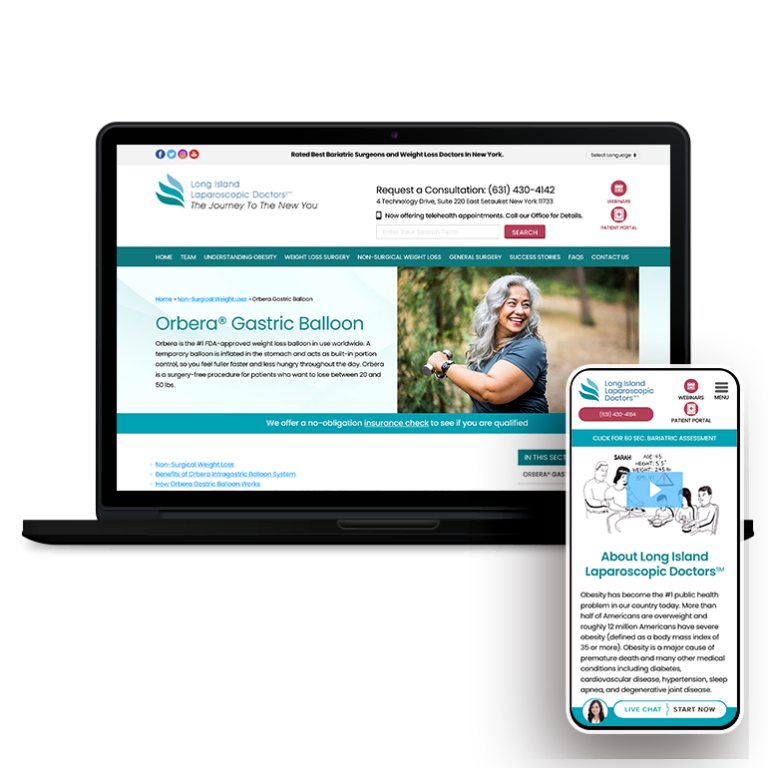
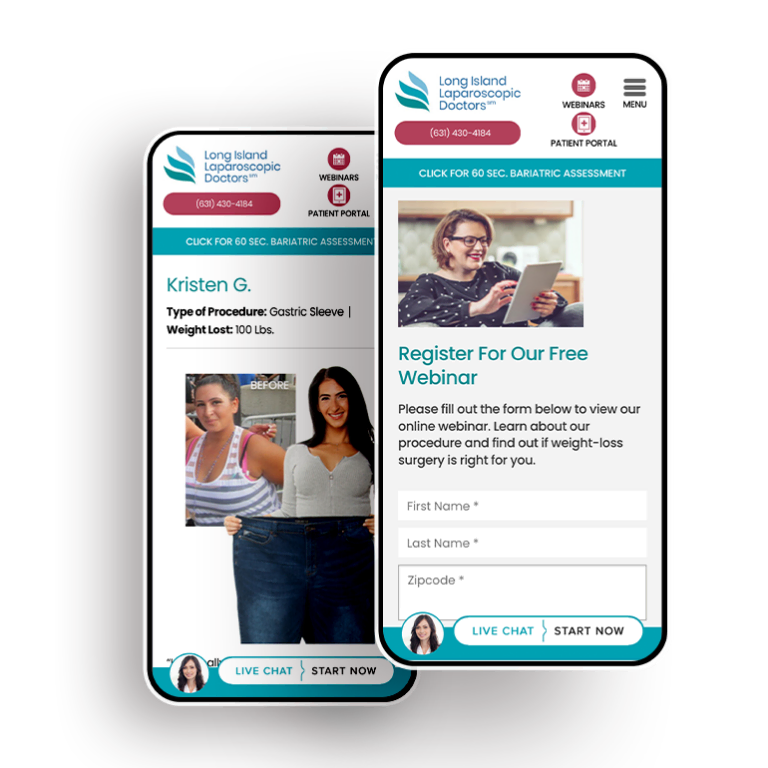

 Smart Design Creates New Patient Opportunities
Smart Design Creates New Patient Opportunities