With pharma marketing spend at an all-time high, medical marketers need to prepare for increasing regulations on the horizon.
According to a study published in JAMA at the beginning of 2019, medical marketing spending has increased by two-thirds in the 20 years between 1997 and 2016. Direct-to-consumer advertising constituted the largest growth area, increasing from $2.1 billion in 1997 to $9.6 billion, or 32 percent of all pharma marketing spending, in 2016.
Meanwhile, FDA violation letters for misleading drug marketing have actually decreased over the last two decades. While 156 such letters were issued in 1997, only 11 were issued in 2016. According to the JAMA authors, “Companies might be producing better materials, but it is also possible that FDA reviewers may be overwhelmed by the massive increase in promotional submissions.”
A growth in medical marketing spend isn’t necessarily a bad thing. After all, marketing can help to increase awareness and reduce stigma associated with certain conditions such as HIV or depression, and it can prompt patients to get appropriate tests or treatments.
However, there’s certainly a negative side to the recent influx of pharma marketing: it can lead to overdiagnosis and overtreatment. Studies show that patient requests for specific medications can have a substantial impact on doctors’ prescribing decisions, even when the medication isn’t actually the best fit for the patient.
Regulations on the Horizon
To protect patients, physicians, and medical marketers alike, it’s only a matter of time until the FDA increases regulations surrounding pharma advertising. Until now, regulatory practices haven’t kept up with the marketing surge.
While the FDA can act when an advertisement makes false claims or violates other statutes, most pharma ads don’t receive a federal stamp of approval before hitting desktops or televisions. Many consumers don’t realize this, so they’re also not aware of the inherent biases in most ads.
For example, while pharma ads on TV may mention side effects in a quick voiceover at the end, most don’t honestly weigh the risks and benefits of the treatment they’re promoting. The ads also don’t mention alternatives for treating a given condition, and they rarely mention the associated treatment cost.
Physicians are meant to act as the liaison between advertisers and patients, steering patients away from treatments that are unnecessary or a poor fit. However, doctors are targeted by marketers, too — this can impact their ability to make unbiased prescription decisions. Due to these concerns, new regulations are certainly on the horizon, and pharma marketers will have to adjust accordingly.
How Pharma Marketers Can Adjust
Moving forward, digital marketing best practices for pharma are bound to change. Fortunately, they’ll be changing for the better, increasing trust in medical marketing while also offering patients and physicians access to the best information possible.
In the future, medical marketers will need to be even more careful about their claims. Claims will not only have to be factually accurate, but acknowledge that medical adds can lead to overdiagnosis and overtreatment of certain conditions. The new regulatory environment will likely require more disclosure surrounding advertising to help consumers discern between physician and advertiser recommendations.
In a similar vein, these advertisements should encourage patients to consider their doctors the first and final point of contact for all of their care decisions. Fortunately, pharma marketers can help to make their advertisements both above-board and effective by making research more available to the public and helping both patients and physicians remain informed about treatment breakthroughs.
















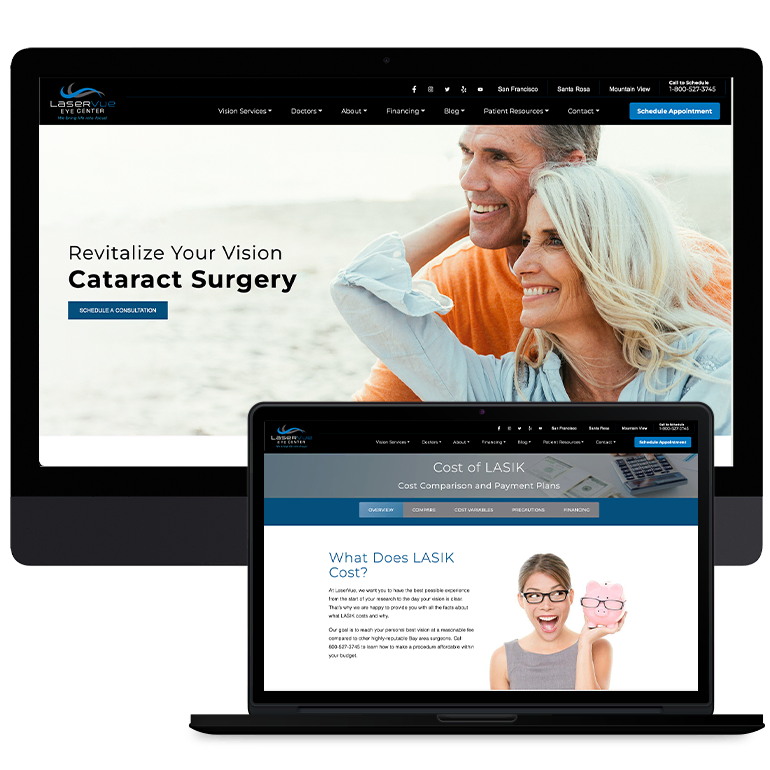



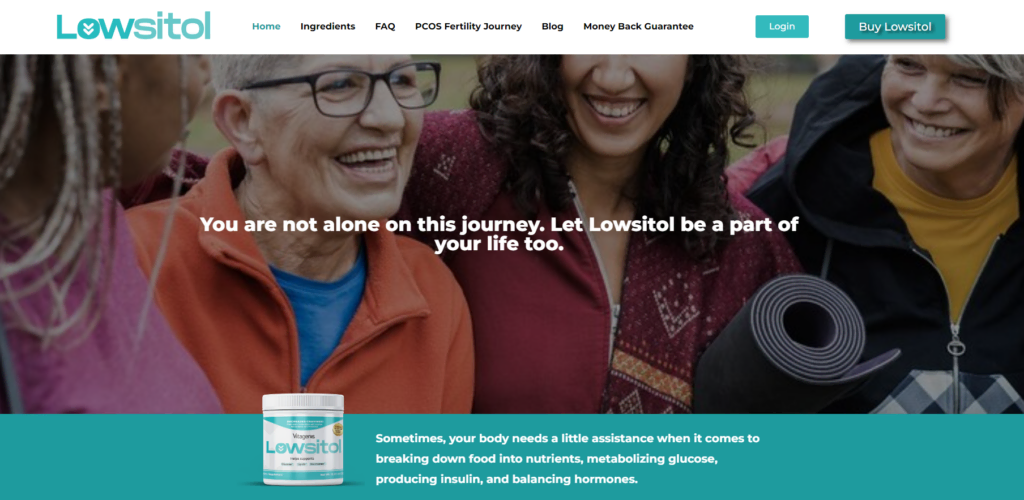
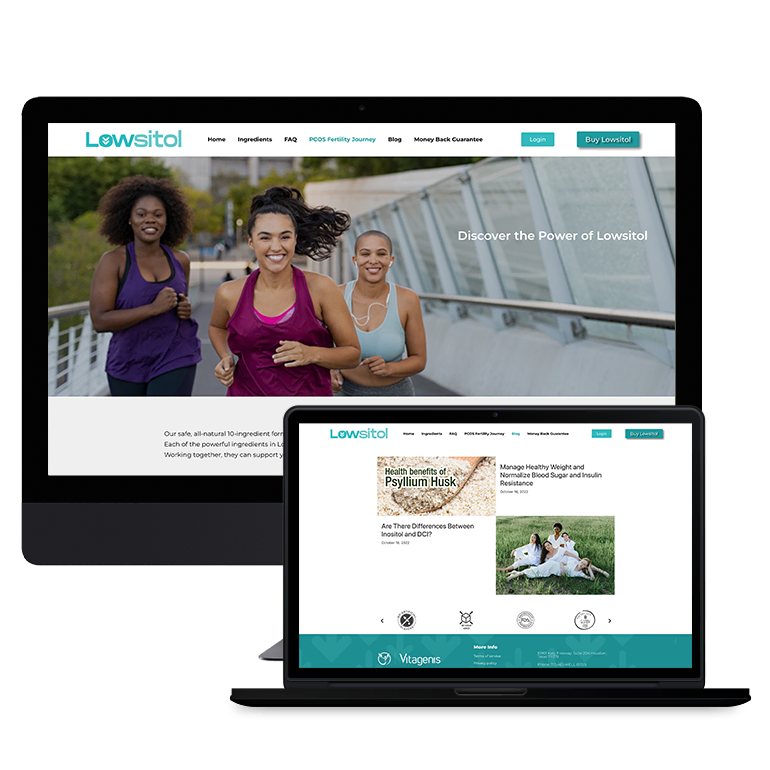
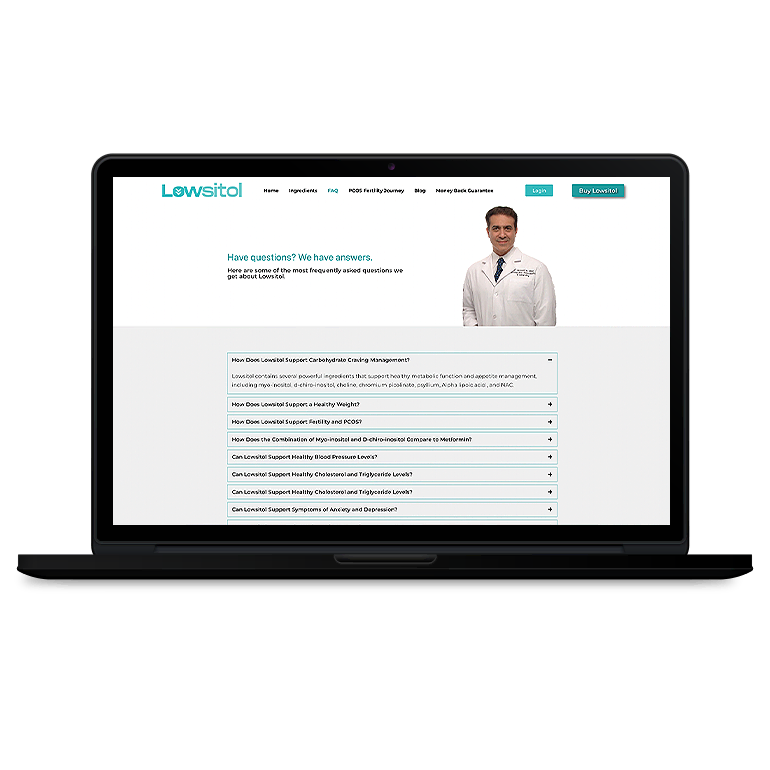










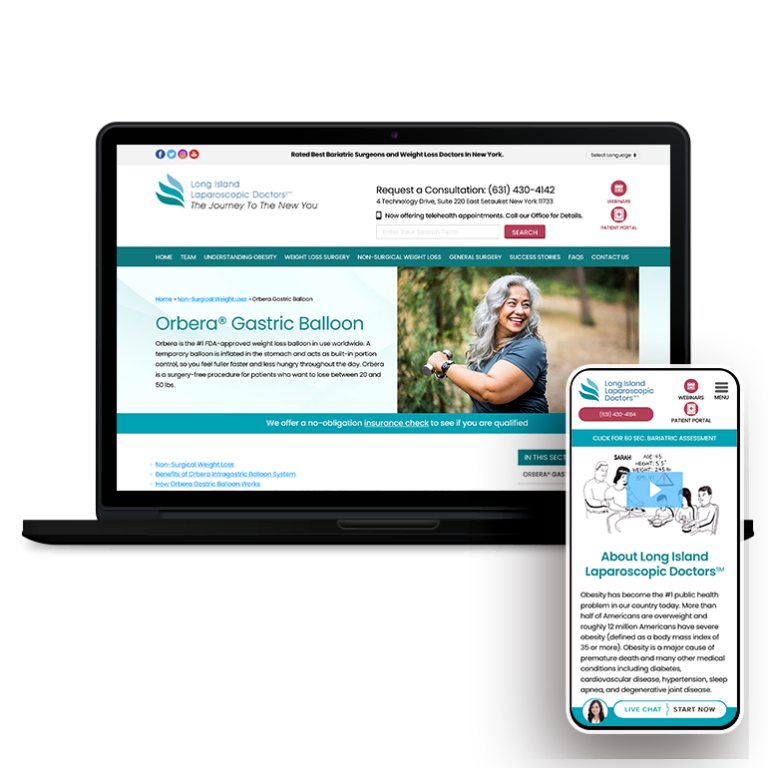
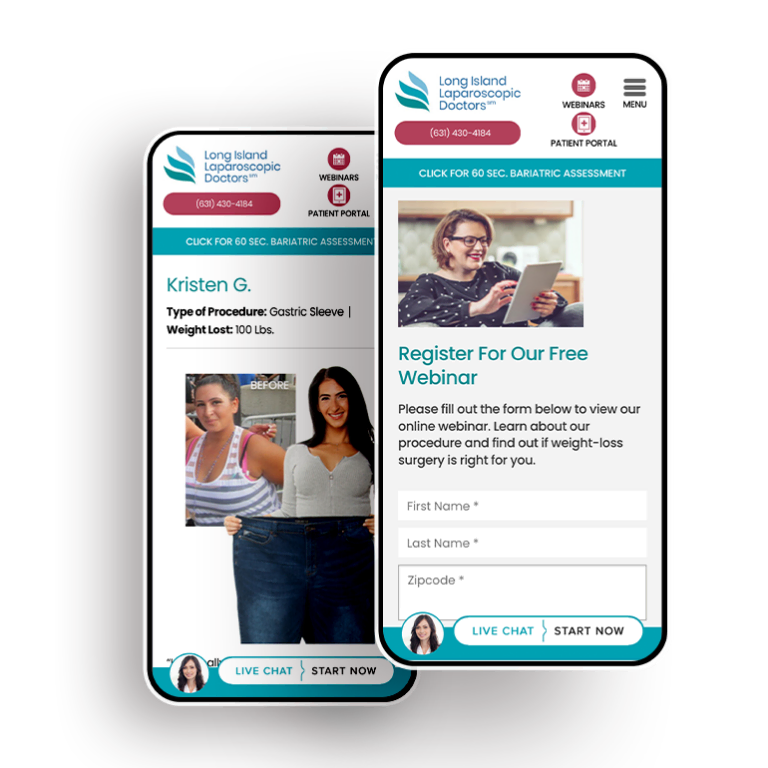
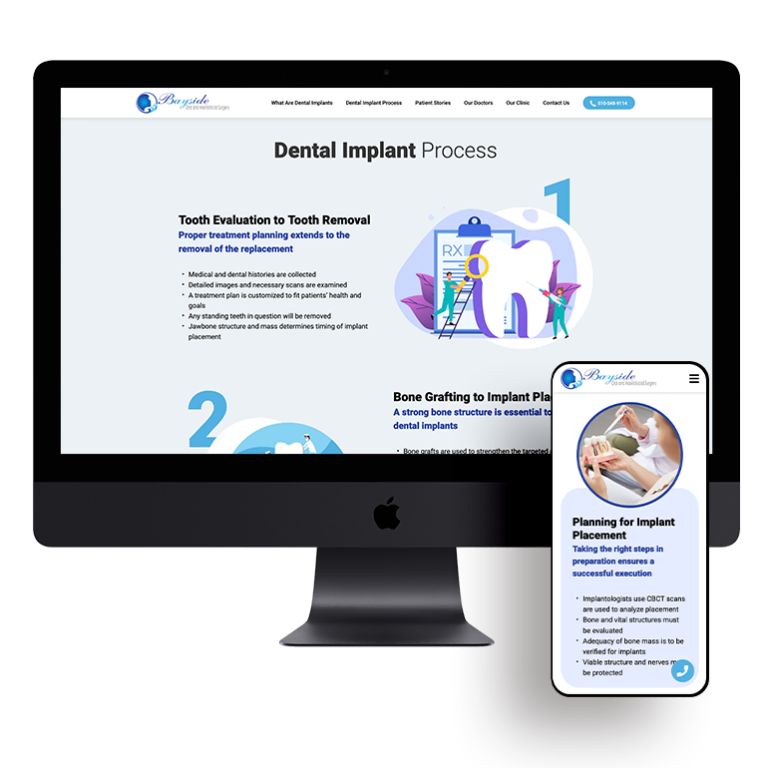
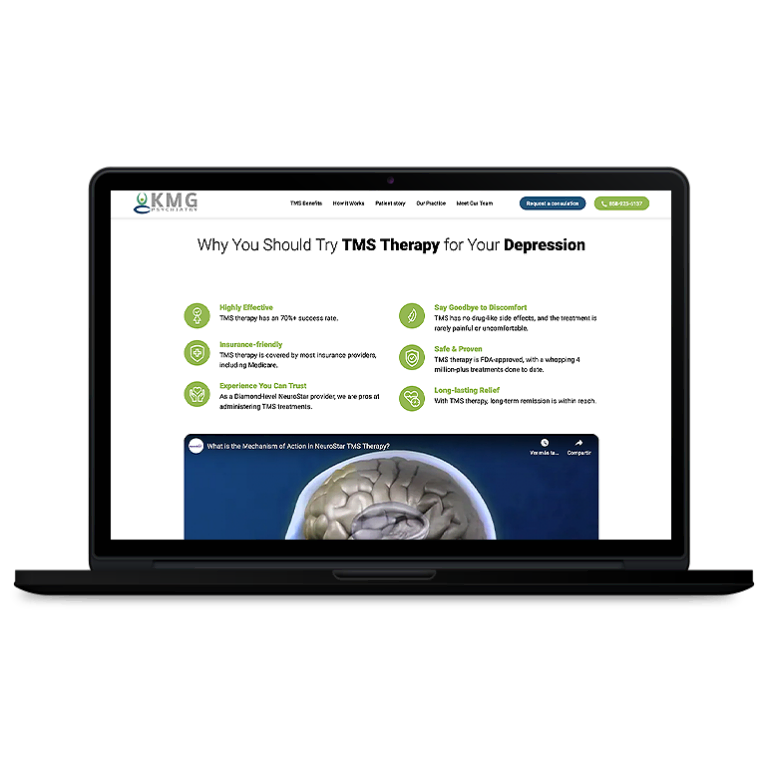
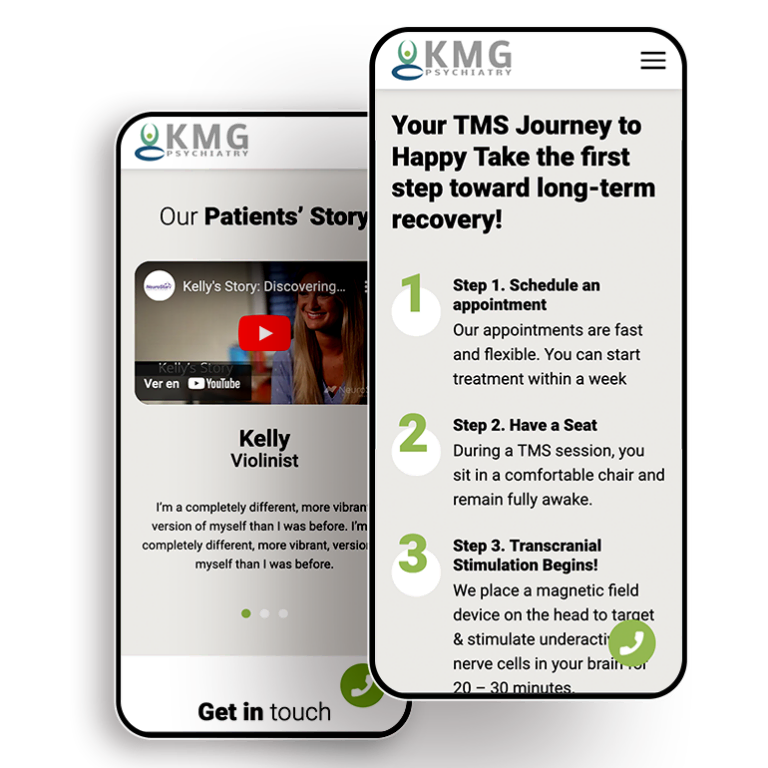
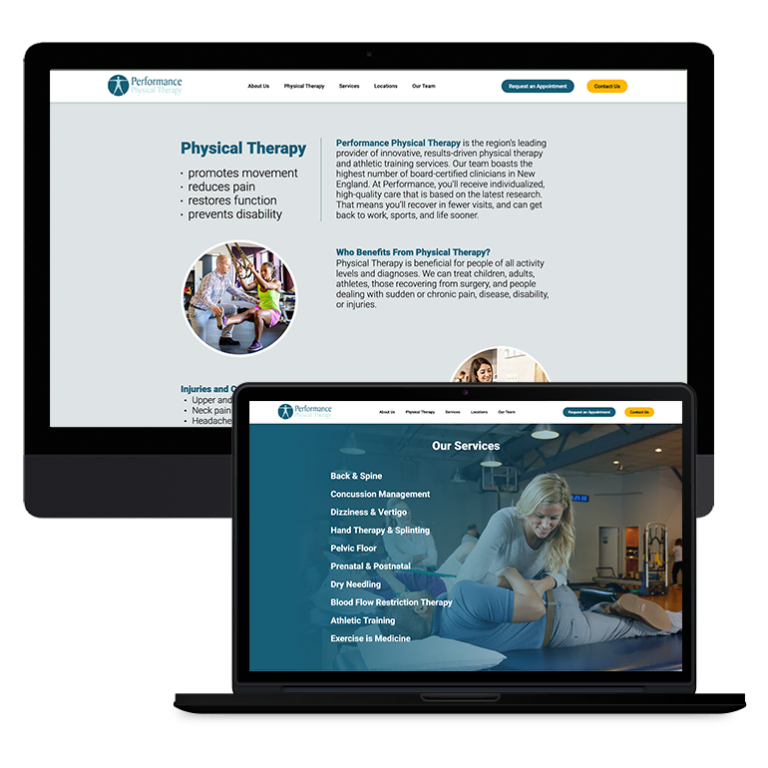

 Smart Design Creates New Patient Opportunities
Smart Design Creates New Patient Opportunities